Why Do I Need to Continue Taking Blood Thinners After Having the Watchman Implant
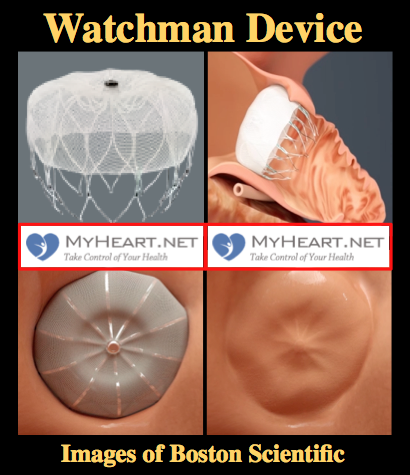
What is the Watchman Device (Also known as Watchman Procedure, Watchman Heart Procedure or Watchman Implant)?
The Watchman Device is a small implant placed in to the heart that can reduce the risk of stroke in patients with atrial fibrillation. Patients with atrial fibrillation are at increased risk of stroke, mainly due to clots that form in a small chamber in the top of the heart known as the left atrial appendage. In the Watchman procedure a small tube is passed up to this appendage through the veins of the leg and the watchman device is deployed, closing off the appendage. For this reason the Watchman procedure with the Watchman Device is known as a form of left atrial appendage occlusion.
The Watchman procedure is a structural heart procedure such as the TAVR procedure for aortic stenosis or the Mitraclip procedure for mitral regurgitation.
How does the Watchman Device reduce stroke risk?
The left atrial appendage is a small sac in the top left chamber of the heart. In people with atrial fibrillation, this sac quivers constantly, the blood in the sac becomes stagnant as a result, and clots can form in it known as left atrial appendage clots. These clots can get loose and travel to the brain leading to strokes. Atrial fibrillation is the most common cause of stroke and the majority of strokes in atrial fibrillation are caused by these appendage clots. Implanting the Watchman Device in to the left atrial appendage basically closes off the appendage and prevents any clots forming inside.
The Watchman Procedure Described in Moving Pictures
In this section we will describe the procedure using images as seen below. The images are as obtained from Boston Scientific Original information videos. The goal here is for patients and healthcare providers who aren't familiar with the Watchman procedure to be able to understand it more easily.
Getting access to the leg vessel
The Watchman Procedure is a minimally invasive procedure performed through the vessels of the leg. Firstly, access to the leg vessel is obtained through a small needle and a wire. The patient will not feel this, as they are asleep.

Transseptal Puncture

A tube is passed up through the vein of the leg, to the right side of the heart. Remember the left atrial appendage is on the left side of the heart. The tube is passed from the right to the left side of the heart by puncturing the wall between the right and left side of the heart known as the inter-atrial septum. This is called a transseptal puncture.
Getting Access to the Appendage
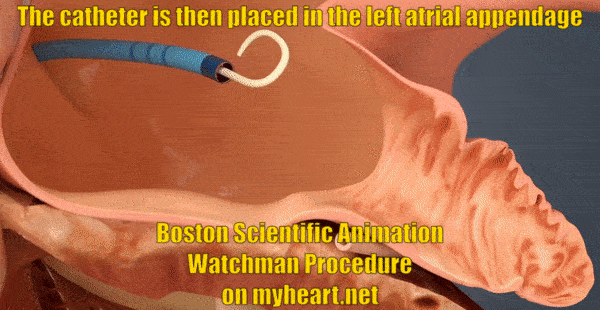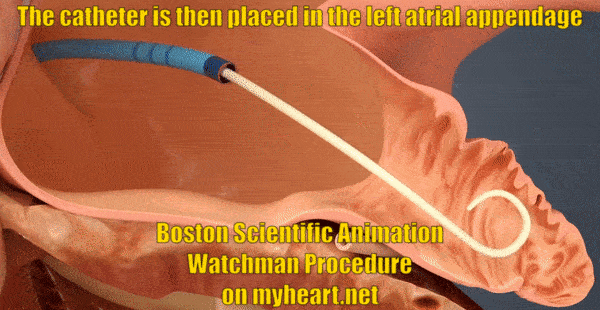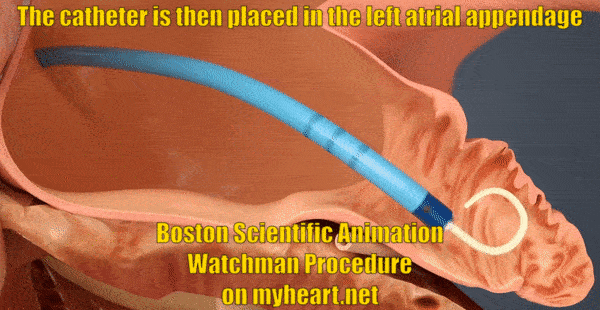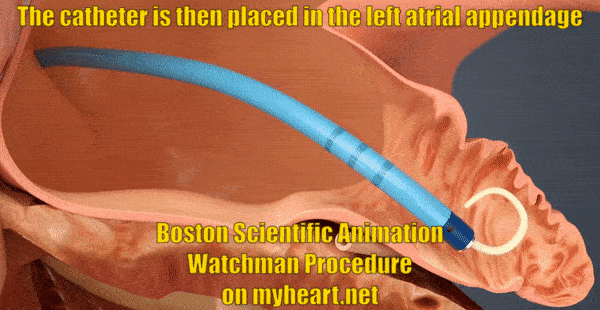
The whole goal of the Watchman Procedure is to close off the left atrial appendage. The appendage is where the clots form that lead to stroke in patients with atrial fibrillation. In this next portion of the procedure, the tube through which we will deliver the Watchman Device is placed in to the appendage. The tube is positioned using a catheter known as a pigtail catheter that is soft and unlikely to cause damage to the heart structures.
Deploying the Watchman Device
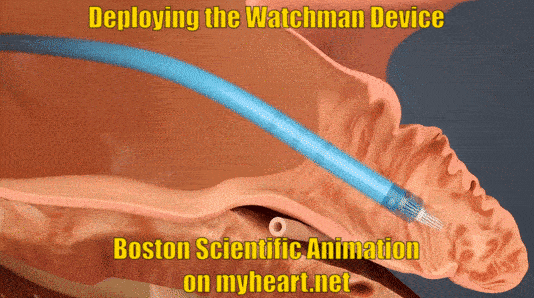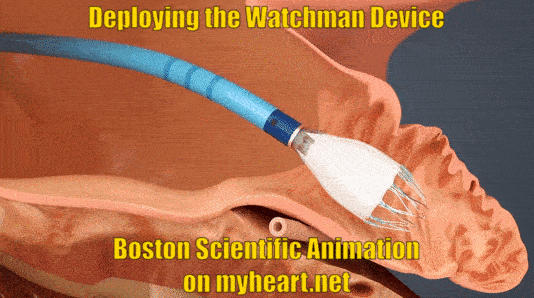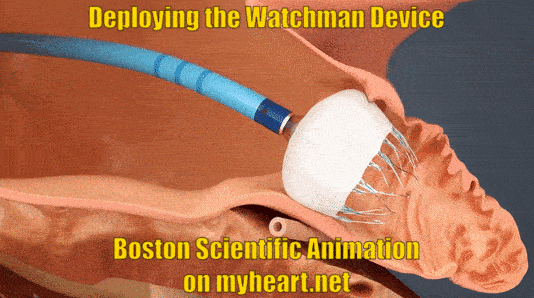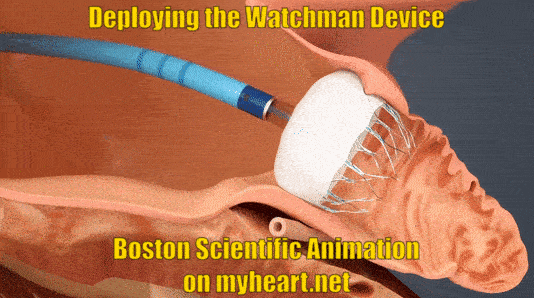
The device is advanced to the appendage through the tube. When in place the tube is slowly pulled backwards and the Watchman Device takes shape in the mouth of the appendage until it is fully expanded in place. At this point a number of checks are performed to ensure the device stability.
Ensuring Watchman Device Stability
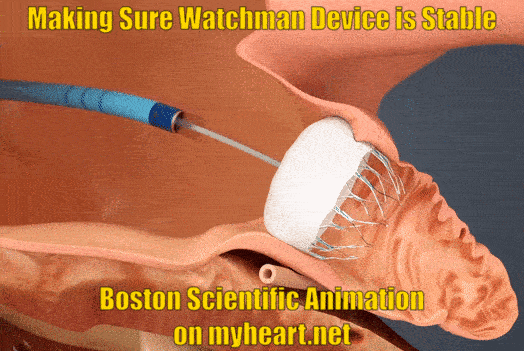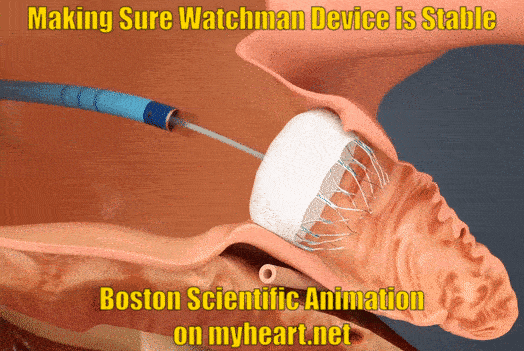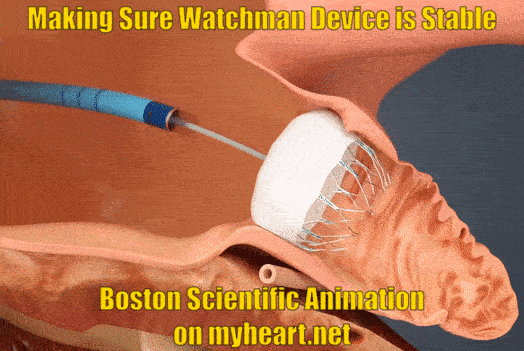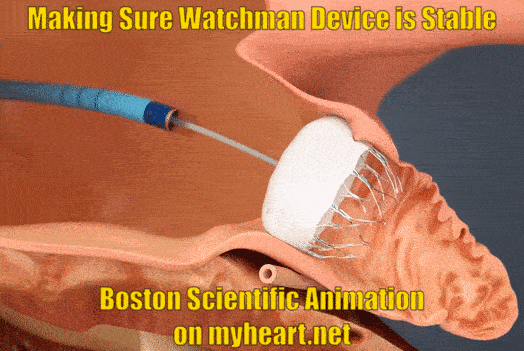
As part of the checks to make sure the device is nicely in place and stable the Watchman device is gently tugged on to make sure is stays in place and isn't loose. This is called a tug test. There are small anchors in the device that keep it in place also.
Releasing the Device
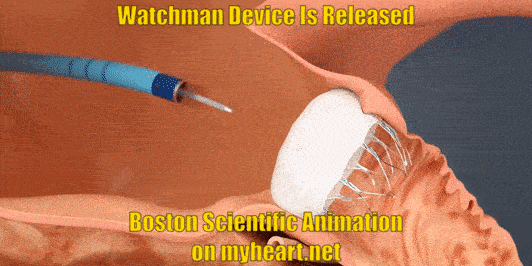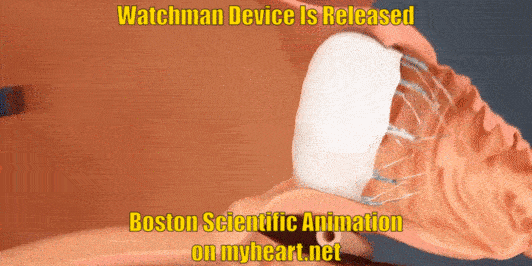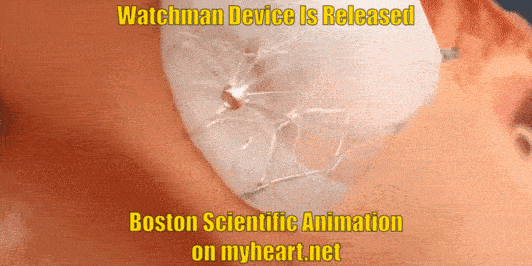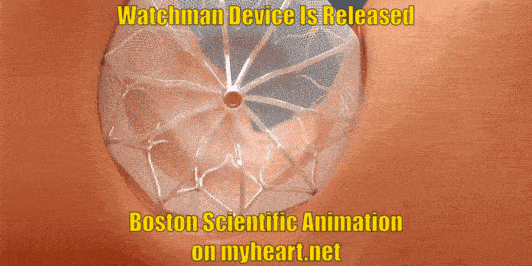
The Watchman Device is still attached to a delivery cord. This cord is removed by gently unscrewing the cord until the device is detached. Of course prior to detaching the device, a number of checks have been performed to ensure that the device is stable and in correct position.
Device Endothelialization

The device is nicely in place and over the next weeks to months a process occurs whereby the body covers the device with its own lining known as Endothelialization. This is great for a few reasons. Firstly, this ensures the device will essentially remain stable lifelong. Secondly, this will further act to prevent clot formation in and around the appendage.
Who should be considered for a Watchman Device?
Historically patients with atrial fibrillation that were considered to be of a high stroke risk were treated with blood thinning medications such as Coumadin / Warfarin to reduce stroke risk. As things stand, use of blood thinner is the preferred treatment for patients with atrial fibrillation. Almost half of patients with atrial fibrillation cannot tolerate the blood thinner. Some of the reasons for this include bleeding issues, concerns for falling, lifestyle issues and inability to tolerate the medication. In such patients, the Watchman Device is felt to be a good alternative to reduce stroke risk.
Who is a Watchman Device Candidate?
Firstly lets look at the official guidelines then we can discuss this in a little more detail in a way that patients can understand. In order to be a candidate for the Watchman Device, the following criteria need all to be fulfilled.
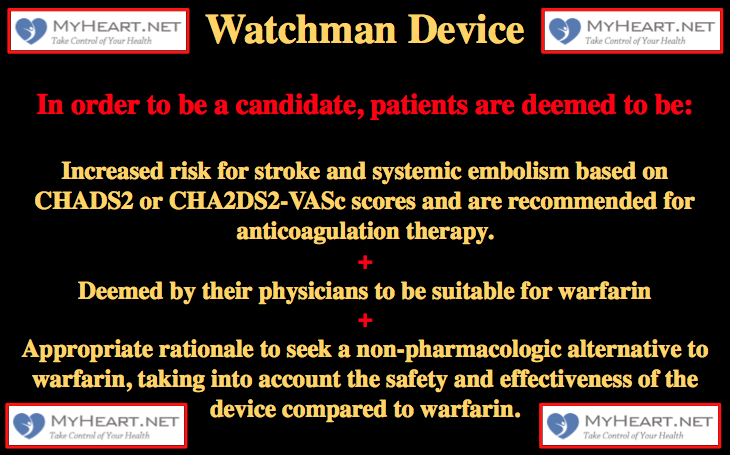
- Increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc scores and are recommended for anticoagulation therapy.
- Deemed by their physicians to be suitable for warfarin
- Appropriate rationale to seek a non-pharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared to warfarin.
Firstly, patients must be at increased risk for stroke. When we are determining stroke risk for atrial fibrillation patients we use scoring systems known as the CHADS2 score or the CHA2DS2-VASc score. To determine these scores we basically put numbers in to the scoring calculator. Let me take you through an example.
C ongestive heart failure – a history of heart failure is 1 point
H ypertension – a history of high BP is 1 point
A ge ≥ 75 – age ≥ 75 is 1 point
D iabetes – a history of diabetes is 1 point
S troke – stroke or TIA history is 2 points

So, if we had a patient that is 75 years old (1 point), and has a history of high blood pressure (1 point) and diabetes (1 point), this gives us 3 points and therefore a CHADS2 score of 3. In general those with a CHADS2 score ≥2 or a CHA2DS2-VASc score ≥3 are considered high risk of stroke and conventionally would be treated with blood thinning medications.
Secondly, patients must be deemed suitable candidates for warfarin. You have to at least be able to tolerate warfarin for 45 days after the procedure during the endothelialization process. You will then need to take another type of blood thinner known as Plavix, or clopidogrel, for another 6 months.
Thirdly, there must be appropriate rationale to seek a non-pharmacologic alternative to warfarin, taking into account the safety and effectiveness of the Watchman Device compared to warfarin. Now remember the first line treatment for atrial fibrillation patients considered at high risk of stroke is the use of blood thinning medications such as warfarin. The Watchman Device is for those who are felt to be unsuitable for long-term treatment with blood thinners. Later in the section "what does a Watchman patient look like" we go through some different scenarios. Examples would be patients who have had a bleed or are at high risk for bleed for various reasons. Or a patient who is at high risk of falling or has a history of falls for whom blood thinners are too dangerous. Or a patient who has a lifestyle incompatible with the use of daily blood thinning medication. Regardless, there must be an appropriate rationale for the patients not being on long-term blood thinning medication.
The Watchman Procedure – Does Appendage Type Matter?
The Watchman Device is designed to cover and essentially eliminate the appendage, basically preventing clots from forming there and reducing stroke risk. The video below shows the three main appendage types. Chicken-wing, broccoli and windsock types. Yes these names appear ridiculous when describing anatomy however they accurately describe the shape of the appendage. The easiest to do is the windsock type, as this is easier to deliver. The chicken wing can sometimes be straightforward and sometimes more difficult. The broccoli is felt to be the most difficult. Regardless successful use of the Watchman Device has been described in all these circumstances.
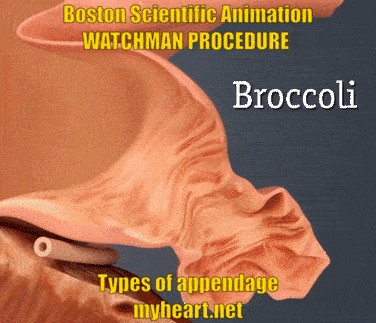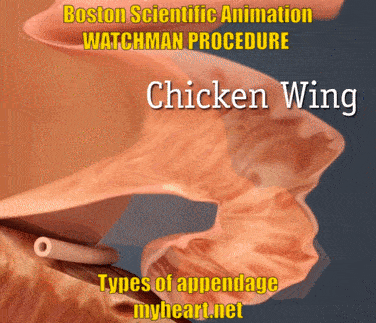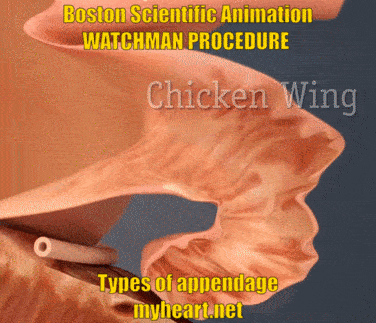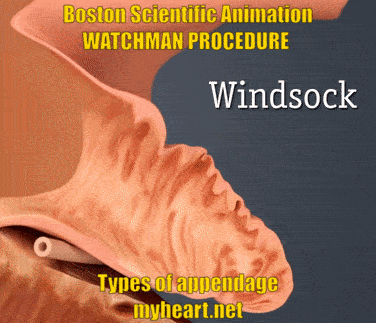
The Watchman Procedure – Does Appendage Size Matter?
There are 2 important things when considering appendage size, the width of the entrance and the length of the whole thing. The width is important, as the chosen device must cover the whole opening to seal off the appendage. The length is important, as it must be long enough to deliver the chosen device. The good thing is the device comes in many sizes. 21mm, 24mm, 27mm, 30mm and 33mm. The length of the appendage must equal the maximum size of the appendage opening to be able to accommodate delivery of the device.
Why don't all patients with atrial fibrillation have the Watchman Device implanted?
The recommendation for the use of the Watchman device is based on evidence gathered over many years and in thousands and thousands of patients. As things stand, the evidence is there only for patients that don't tolerate blood thinner. There is no good evidence currently that atrial fibrillation who are doing well taking blood thinning medication should have the Watchman Device implanted.
Who Should Not Get a Watchman Device?
Even in those patients who have already fulfilled the above criteria, the Watchman Device should not be used in the following groups of patients.
Intracardiac thrombus is visualized by echocardiographic imaging . This means that patients who have been found to have clots in the heart should not have the device. This is because the Watchman Procedure itself would be associated with a high risk of the clot dislodging and a stroke or other major complication.
An atrial septal defect repair or closure device or a patent foramen ovale repair or closure device is present . In patients that have had a history of a hole in the heart and a device already used to close it, the Watchman Procedure is not typically a good idea as the existing device will simply get in the way of the procedure.
The LAA anatomy will not accommodate a device . Remember the Watchman Device is designed for placement in the left atrial appendage. Although in most cases the size and shape of the appendage is ok, in some cases it is not able to accommodate the device. This should be known prior to the procedure.
Miscellaneous reasons . Reasons may include inability to use a TEE probe for imaging, the presence of active bleeding or infection, anatomy that does not allow catheters to be used appropriately, the patient has a hypersensitivity to the components of the device, etc. Patients should typically be able to use aspirin or Plavix.
What are the proposed advantages of the Watchman Device Implant?
Patients with the Watchman Device can typically stop strong blood thinning medication such as Coumadin 45 days after the device is implanted therefore decreasing the chance of bleeding complications and the need to take daily medicines. There is the reduced cost associated with not needing to take a lifetime worth of blood thinner.
Who performs the Watchman Procedure and where should you have it done?
The Watchman Procedure is a unique procedure in that it requires a unique approach called a heart team with structural heart training. A heart team for the Watchman Device typically includes interventional cardiologists, electrophysiologists, imaging specialists and other specialists to work in combination. The procedural selection, planning, and performance rely on these specialists working together. For this reason the Watchman Procedure should only be performed in a place with an established heart team in place and strong individual programs in all these aspects. Every part of the procedure is critical, including the pre-procedural selection and imaging, and all contribute to achieving a positive outcome. The correct team and approach leads to reduced complications and improved outcomes.
How long does a Watchman Device last?
Thousands and thousands of Watchman Devices have been implanted over the last decade worldwide and data regarding the durability of the Watchman Implant is accumulating. Shortly after placement the body's own lining covers the device, essentially incorporating it into the body. Essentially if correctly done, the procedure should last lifelong with regards to occlusion of the left atrial appendage.
What tests are required for the Watchman Procedure?
Initially the Watchman Heart team evaluates each patient with atrial fibrillation and determines the suitability on a case-by-case basis. Once that has been determined then other tests are performed to assess suitability for implantation of the Watchman Device.
Typically an ultrasound scan of the heart known as a TEE is performed to assess the left atrial appendage and to help select sizing of the Watchman Device.
In many cases CT scanning may be performed to allow assessment of the heart to determine left atrial appendage type and size, and also to assess the approach taken.
How long will a Watchman Device patient have to stay in the hospital after Watchman Implantation?
As Watchman technology and experience continues to evolve the length of hospital stays are growing shorted and shorter. Typically patients leave the hospital the day after the procedure.
What medications need to be taken after a Watchman Device Procedure?
The point of the Watchman Device implant is to avoid the need for blood thinning medication. As things stand, the blood thinning medication Coumadin needs to be taken for 45 days after the procedure. This is because it is felt that until this time the device has not been covered by the body's own lining and so there is still risk of clot formation. In the years to come, the need for strong blood thinner after the procedure may disappear all together.
What are limitations After a Watchman Procedure?
The limitations are minimal. In terms of activity you may be told to take it easy for a day or so to prevent bleeding issues from the vessels of the leg the device was placed through. There are the usual limitations and care needed for patients that take blood thinner, in that anything with more than an ordinary risk of bleeding should be avoided until the blood thinner can be stopped.
What is the Watchman Device made out of?
The Watchman Device is made out of a metallic frame that is then covered with a thin layer of fabric. It is extremely lightweight. The frame is made out of a nitinol alloy that is very common with cardiac implants. The fabric is made from Polyethylene Terephthalate (PET) and is attached by tiny sutures.
Can patients have MRI scans or go in metal detectors with a Watchman Device?
Patients can safely go through metal detectors. Patients can have MRI scans under the conditions listed on their implant cards. If patients are to undergo MRI scans then they should first hand their card to the radiologist and ensure the conditions are met.
Important After Procedure Details
Medications
Remember one of the key goals of the procedure is to avoid the long-term use of often-undesirable blood thinning medications such as warfarin. As things currently stand patients will need to stay on warfarin for 45 days post procedure. The INR range is between 2-3. Patients will continue to stay on aspirin 81mg during this time. In most cases warfarin can be stopped at the 45-day point. It is possible in the future that there will be no need for warfarin after the procedure at all.
In general, after the Warfarin has been stopped, the aspirin dose will be increased to 325 mg and Plavix 75 mg daily is started also until the 6-month point. After 6-months usually aspirin alone is given at 325 mg daily.
Tests
At 45 days after the procedure a TEE test is performed; remember this is the small camera inserted in to the food pipe to get close images of the heart. The test is performed to ensure the Watchman device remains well positioned and continues to do its job.
Risks of the Watchman Procedure
As with any invasive procedure there are risks associated. The good news is that the risk of complication is small, and has continued to decrease as the cardiologists performing and planning the procedure have continued to gain experience and make the procedure safer.
Damage to the leg vessels – Remember the procedure is carried out through the vessels of the leg and there is a small chance of damage here. If there is damage, then this can usually be taken care of without too much difficulty.
Infection – Antibiotics are given appropriately to minimize any risk. Infection could theoretically occur at the entrance site on the leg or on the device itself; this would be extremely rare, however.
Damage to the heart structures – The heart structures are often thin and there is always a chance of causing tears in the heart linings and escape of fluid in to the sac around the heart, known as a pericardial effusion. In some cases this would require emergency surgery or at least insertion of a drain in to the sac around the heart. In experienced hands this is a rare complication.
Device Embolization – The Watchman Device, if not seated properly, could theoretically come loose and essentially float freely until it became trapped in a heart structure. This would typically require cardiac surgery to retrieve the device. The good news is that with an extensive procedural checklist in place to ensure the device is stable, this complication hardly ever occurs.
Real Life Experience of the Watchman Procedure in the United States
Although clinical trials gave us important data that led to approval of the Watchman Procedure by the FDA, it's important to know the real life experience that can give us important insight into the safety and effectiveness of the procedure.
A recent paper was published entitled "Post-FDA Approval, Initial US Clinical Experience with Watchman Left Atrial Appendage Closure for Stroke Prevention in Atrial Fibrillation."
The paper reported on the outcomes of almost 4000 cases in the United States. The implantation of the Watchman Device was successful 96% of cases. The procedure took around 50 minutes on average. The rate of fluid build up around the heart was 1% and of that 1% one-third required emergency surgery. The rate of the device coming loose was very low at 0.25%. In total there were only 3 procedure related deaths, which is 0.078%.
These findings tell us that since FDA approval of the Watchman Procedure, it has in general been performed successfully in an efficient manner and the rate of complication is remarkably low.
Source: https://myheart.net/articles/watchman-device-explained-and-faqs-answered-by-a-cardiologist/
0 Response to "Why Do I Need to Continue Taking Blood Thinners After Having the Watchman Implant"
Post a Comment